What causes aseptic loosening of joint prostheses
Introduction:
With the development of surgical techniques and materials, surgical failure due to infection, fracture and dislocation has become relatively rare. Aseptic loosening of the prosthesis caused by a variety of factors is a common cause of joint arthroplasty failure. Aseptic loosening refers to the loosening of the artificial joint from the bone interface without infection or trauma. The number of joint replacement surgery is increasing year by year, and the number of revision surgery is increasing. It is particularly urgent to explore the root cause of aseptic loosening. This article will analyze the core causes of aseptic loosening in detail, and focus on the mechanism and influencing factors of implant aseptic loosening caused by the host biological response caused by wear particles, in order to provide theoretical basis for the treatment and prevention of aseptic loosening.
Detailed analysis of the pathogenesis of aseptic loosening
The causes of aseptic loosening are quite complex, and it is difficult to fully elaborate through a single theory. When discussing its pathogenesis, it usually involves two aspects: mechanical theory and biological theory. The mechanical theory mainly includes prosthesis micromotion, stress shielding effect and high hydraulic force. In terms of biological theory, the inflammatory response induced by wear particles is considered to be a key factor leading to aseptic loosening.
1.Mechanical factors
1.1 Prosthesis micromovement:
Prosthesis micromovement refers to small amplitude movement, vibration or sliding between two mechanically connected structures (such as prosthesis and bone tissue) under load. The stability of implant bonding to bone tissue is the key to prevent aseptic loosening. Therefore, the design of implants should ensure that they can form a close and stable bond with bone tissue. The design of the prosthesis may not completely avoid the occurrence of micromovement. For example, the structure of the junction, the choice of material, and the processing mode, among others, may influence the degree of freting.
1.2 Lower limb alignment:
The lower limb alignment extends from the center of the femoral head to the center of the ankle joint, ideally through the center of the knee joint or slightly to the medial side. Restoring and maintaining this neutral alignment is regarded as one of the key criteria to obtain excellent clinical results in total knee arthroplasty. By ensuring the neutral state of the postoperative force line, the stress of the knee joint can be evenly distributed, so as to effectively prolong the service life of the prosthesis. The selection of the implant Angle is very important: if the implant is placed in the flexion position, it will cause a direct impact between the anterior intermalleolus and the central support structure of the prosthesis during the knee extension movement, resulting in significant contact stress. This high stress not only accelerates the wear process of the prosthesis, but also induces osteolysis around the prosthesis once the particles from wear spread into the joint cavity, which may eventually lead to prosthesis loosening.
1.3 Stress shielding effect:
Stress shielding refers to the change in stress distribution caused by the inconsistency between the stiffness of the prosthesis material and the surrounding bone tissue, which is closely related to the physiological conditions. Specifically, a prosthesis with higher stiffness will bear more loading stress, while a prosthesis with lower stiffness will bear relatively less. Stress shielding phenomenon can cause bone loss, which may lead to prosthesis loosening. Bone metabolizing cells have the ability to regulate bone growth and resorption by sensing appropriate mechanical stimuli. Within the physiological range, moderate low-intensity mechanical stimulation has a positive effect on osteogenesis, and this promoting effect is closely related to the primary cilia on the surface of BM-Mscs. However, the stress shielding effect after prosthesis implantation will destroy the mechanical environment of bone tissue and make it unable to feel the appropriate mechanical stimulation, which leads to the reduction of osteoblast generation. This chain reaction may eventually trigger prosthesis loosening.
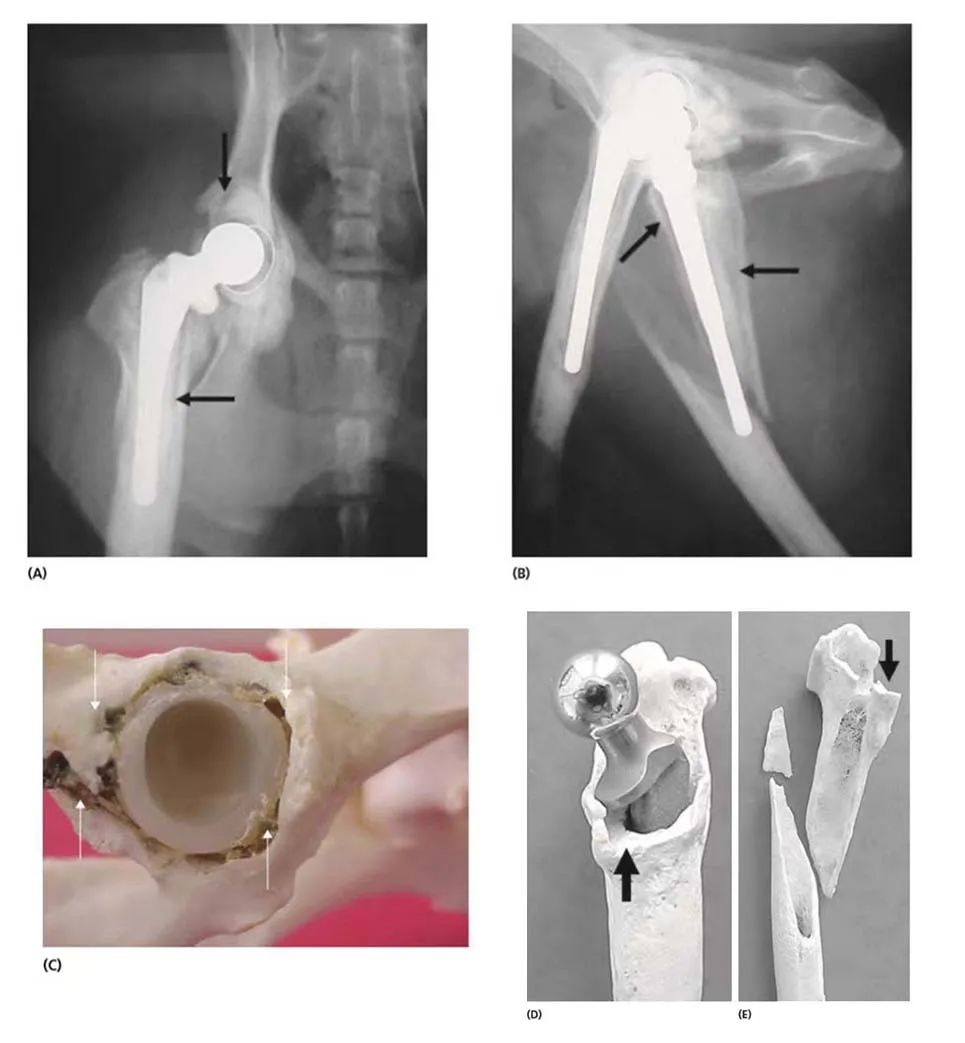
Aseptic loosening and femoral fracture 11 years after cemented hip replacement
2.Biological factors (wear particles)
2.1 Formation of wear particles
Abrasive wear: When two materials with different mechanical properties come into contact, the harder material creates microscopic grooves in the softer material, thus releasing wear particles. This wear is also seen when a third body material (such as cement) is embedded in a softer material (such as a polyethylene liner), causing grinding wear of the femoral head, producing a larger concave and convex surface, leading to further increased abrasive wear.
Adhesive wear: When an intermolecular bond is formed between two materials and subjected to mechanical forces, the difference in strength causes the weaker intermolecular bond to break, resulting in adhesive wear. For example, in hip arthroplasty (THR), where the femoral head is attached to a polyethylene pad, intermolecular bonds form at the junction of these two opposing materials, and if this bond is stronger than the cohesive strength of polyethylene, it may cause portions of the polyethylene pad to be pulled off the femoral head, forming adhesive wear particles.
Fatigue wear: Fatigue wear refers to the cyclic contact stress and deformation of the surface caused by the action of periodic load when the two contact surfaces are subjected to rolling or rolling sliding composite friction, thus causing the material to crack and peel off micropieces or particles.
2.2 Biological effects of wear particles
Wear particles generated by artificial joint prostheses are released at the bone-implant interface and trigger a series of biological reactions, leading to osteolysis and aseptic loosening.
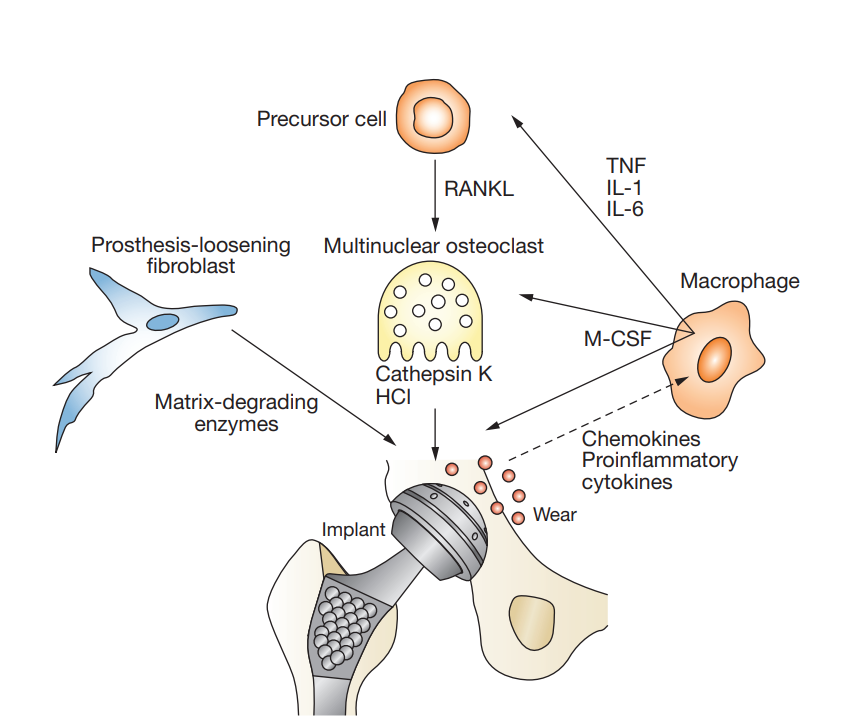
Effect of wear particles on macrophages: Wear particles, such as metal particles, ultra-high molecular weight polyethylene (UHMWPE) particles, and bone cement particles, can activate macrophages. After recognizing and engulfing wear particles, macrophages will be activated and release a series of inflammatory factors, such as TNF-α and IL-6, which participate in the local inflammatory response and may promote the occurrence of adverse reactions such as aseptic loosening and osteolysis. At the same time, wear particles may also affect the survival, phagocytosis and other cellular functions of macrophages, thereby further interfering with the body’s immune response and tissue repair process.
Effect of wear particles on osteoclasts: The role of osteoclasts is to degrade the absorbed bone matrix as well as bone minerals and expel them into the blood as Ca. Due to the stimulation of wear particles, the secretion of cytokines and chemokines around the prosthesis is increased. They can activate NF-κB and lead to the enhancement of osteoclast function, while the activity of osteoblasts may be inhibited, resulting in the balance between bone formation and bone resorption being broken. Long-term bone resorption is greater than bone formation, which will lead to decreased bone mass and bone density, and then cause bone metabolism disorders such as osteoporosis. In the field of orthopedic implants, this disorder of bone metabolism may also cause complications such as prosthesis loosening, which may affect the stability and service life of implants.
The effect of wear particles on osteoblasts: the main role of osteoblasts is to promote bone formation and reconstruction, promote calcium deposition to bone, so that bone tissue density increases and texture becomes hard. Wear particles themselves have certain toxicity, and osteoblasts can phagocytose wear particles with a diameter <5 μm, which has potential adverse effects on cell viability, proliferation, and function. When wear particles are engulfed by osteoblasts, their own toxicity will lead to the death of osteoblasts, release a series of inflammatory substances, and trigger an inflammatory response, which will affect the function and survival of other normal osteoblasts.
Effect of wear particles on fibroblasts: Wear particles acting on fibroblasts can increase the fibrotic process and promote the formation of the fibrous limiting membrane around the prosthesis. The formation of this fibrous limiting membrane may be an important factor in prosthesis loosening. Fibroblasts can also directly phagocytize wear particles with small diameter, produce and release cathepsin K, a large number of osteolytic mediators and inflammatory factors, and may indirectly and directly promote collagen dissolution and bone destruction around the prosthesis.
Conclusion:
In recent years, joint replacement has been widely used worldwide and has significantly improved the symptoms and quality of life of patients. However, aseptic prosthesis loosening is one of the important factors affecting the prognosis of patients, and its mechanism is complex, involving wear particles, autophagy, stress shielding and other aspects. Understanding the mechanism of prosthesis loosening provides an important reference for prevention and treatment. However, with the deepening of research and the continuous improvement of prosthesis materials, we have reasons to believe that the problem of aseptic prosthesis loosening will eventually be overcome, and joint arthroplasty will usher in a broader development prospect.
